|
#1
|
||||
|
||||
|
Алгоритм обследования и лечения на скрытый железодефицит
Вариант алгоритма обследования на скрытый железодефицит (если нет возможности сделать ферритин, то можно использовать % насыщения трансферрина железом: железо/ОЖСС < 0.16 - железодефицит - препарат железа на 3 мес.; железо/оЖСС < 0.25 - железодефицит? - пробное назначение железа)
Из Evaluation and Treatment of Iron Deficiency in Adults Nutr Clin Care.2002;5:220–224 |
|
#2
|
||||
|
||||
|
Еще некоторые размышления по поводу диагностики железодефицита:
It is well established that in healthy people, the overwhelming majority of those seeking help for hair loss, serum ferritin provides the most reliable estimate of iron deficiency in the absence of bone-marrow iron-staining data1 but what parameter should the clinician use in patients with hair loss? To address this issue we need to inquire how laboratory reference ranges for serum ferritin and hemoglobin (Hb) were derived. In 2001, Rushton et al questioned the use of a lower reference limit for Hb, red blood cell count, and serum ferritin in menstruating women, and suggested that these anomalies were a result of sampling populations considered normal but containing a large proportion of female patients who were iron deficient. This hypothesis was supported by a large-scale US study that showed 39% of women had a serum ferritin level below the lowest male value, whereas 38% of women were iron deficient using a transferrin saturation level of below 20%, a recognized indicator of deficiency. Bone-marrow iron staining correlates with serum ferritin concentration in individuals with an erythrocyte sedimentation rate of less than 10 mm/h. These studies show a wide range of serum ferritin values corresponding with an absence of iron staining in bone marrow. Guyatt et al4 found a serum ferritin concentration of 50 μg/L was associated with a 50% chance of an absence of iron in bone marrow. From the data of Puolakka5 the calculated 99% confidence limit for bone-marrow iron staining is a serum ferritin greater than 70 μg/L. Clearly, using the current lower reference limits derived from populations containing a large proportion of iron-deficient individuals leaves the clinician believing their patient is iron replete. This quandary affects the entry and completion points in designing clinical trials investigating iron deficiency and hair loss. For example, one group used their laboratory's parameter for serum ferritin of less than 20 μg/L to define iron deficiency in 5 patients with unexplained chronic telogen effluvium. They then claimed that because these 5 patients had a serum ferritin level above this limit (>20 μg/L) there was no association between iron and unexplained hair shedding. We would question their conclusion. To our knowledge, there is no published evidence supporting a lower biological need for serum ferritin or Hb in menstruating women compared with their male counterparts, neither is there evidence for lower Hb concentration in any other mammal including the menstruating Old World primates. No other mammal shows a sexual dimorphism in Hb or iron status; why should human beings be the exception? Consequently, we would suggest using a serum ferritin greater than 70 μg/L when considering whether or not to instigate iron therapy in unexplained chronic telogen effluvium. Из Iron and hair loss in women; what is deficiency? This is the real question! J Am Acad Dermatol. 2007 Mar;56(3):518-9 Фрагмент ответа на этот комментарий: Many laboratories use serum ferritin concentrations of 10 to 15 ng/mL as the lower limits of normal. This yields only a sensitivity of 59% and a specificity of 99% for diagnosing iron deficiency. Using a cutoff of 41 ng/mL yields a sensitivity of 98% and a specificity of 98% for diagnosing iron deficiency. Dr Rushton and colleagues cite a study that showed that the calculated 99% confidence limit for bone-marrow iron staining is a serum ferritin level greater than 70 ng/mL.3 Our own anecdotal experience is that treatment for many forms of hair loss is enhanced when patients maintain a serum ferritin concentration greater than 70 ng/mL. In our clinic, when a patient does not have anemia (ie, the patient has a normal hemoglobin and hematocrit level) but has a serum ferritin concentration less than 70 ng/mL, we call this condition ‘‘nonanemic iron deficiency.’’ J Am Acad Dermatol. 2007 Mar;56(3): 519 |
|
#3
|
||||
|
||||
|
Еще некоторые размышления по поводу диагностики железодефицита:
It is well established that in healthy people, the overwhelming majority of those seeking help for hair loss, serum ferritin provides the most reliable estimate of iron deficiency in the absence of bone-marrow iron-staining data1 but what parameter should the clinician use in patients with hair loss? To address this issue we need to inquire how laboratory reference ranges for serum ferritin and hemoglobin (Hb) were derived. In 2001, Rushton et al questioned the use of a lower reference limit for Hb, red blood cell count, and serum ferritin in menstruating women, and suggested that these anomalies were a result of sampling populations considered normal but containing a large proportion of female patients who were iron deficient. This hypothesis was supported by a large-scale US study that showed 39% of women had a serum ferritin level below the lowest male value, whereas 38% of women were iron deficient using a transferrin saturation level of below 20%, a recognized indicator of deficiency. Bone-marrow iron staining correlates with serum ferritin concentration in individuals with an erythrocyte sedimentation rate of less than 10 mm/h. These studies show a wide range of serum ferritin values corresponding with an absence of iron staining in bone marrow. Guyatt et al4 found a serum ferritin concentration of 50 μg/L was associated with a 50% chance of an absence of iron in bone marrow. From the data of Puolakka5 the calculated 99% confidence limit for bone-marrow iron staining is a serum ferritin greater than 70 μg/L. Clearly, using the current lower reference limits derived from populations containing a large proportion of iron-deficient individuals leaves the clinician believing their patient is iron replete. This quandary affects the entry and completion points in designing clinical trials investigating iron deficiency and hair loss. For example, one group used their laboratory's parameter for serum ferritin of less than 20 μg/L to define iron deficiency in 5 patients with unexplained chronic telogen effluvium. They then claimed that because these 5 patients had a serum ferritin level above this limit (>20 μg/L) there was no association between iron and unexplained hair shedding. We would question their conclusion. To our knowledge, there is no published evidence supporting a lower biological need for serum ferritin or Hb in menstruating women compared with their male counterparts, neither is there evidence for lower Hb concentration in any other mammal including the menstruating Old World primates. No other mammal shows a sexual dimorphism in Hb or iron status; why should human beings be the exception? Consequently, we would suggest using a serum ferritin greater than 70 μg/L when considering whether or not to instigate iron therapy in unexplained chronic telogen effluvium. Из Iron and hair loss in women; what is deficiency? This is the real question! J Am Acad Dermatol. 2007 Mar;56(3):518-9 Фрагмент ответа на этот комментарий: Many laboratories use serum ferritin concentrations of 10 to 15 ng/mL as the lower limits of normal. This yields only a sensitivity of 59% and a specificity of 99% for diagnosing iron deficiency. Using a cutoff of 41 ng/mL yields a sensitivity of 98% and a specificity of 98% for diagnosing iron deficiency. Dr Rushton and colleagues cite a study that showed that the calculated 99% confidence limit for bone-marrow iron staining is a serum ferritin level greater than 70 ng/mL. Our own anecdotal experience is that treatment for many forms of hair loss is enhanced when patients maintain a serum ferritin concentration greater than 70 ng/mL. In our clinic, when a patient does not have anemia (ie, the patient has a normal hemoglobin and hematocrit level) but has a serum ferritin concentration less than 70 ng/mL, we call this condition ‘‘nonanemic iron deficiency.’’ J Am Acad Dermatol. 2007 Mar;56(3): 519 |
|
#4
|
||||
|
||||
|
Может быть, все же упомянуть где-то, что анемия это клинический синдром, который может быть следствием тяжелых болезней - онкологии (обычно в старшем возрасте, в том числе, и гематологической - в позапрошлом году в нашей больнице был бум миеломной болезни, которая долгое время считалась ЖДА (с ним и сочеталась) и лечилась по принципу "у вас железодефицит, пейте железо, ешьте мясо", если бы не обратили внимание на СОЭ, не взяли белок и не начали это дело раскручивать), язвенной болезни и т.д.
|
|
#5
|
||||
|
||||
|
Понравилось
Treatment of anemia due to iron deficiency
Stanley L Schrier, MD UpToDate performs a continuous review of over 375 journals and other resources. Updates are added as important new information is published. The literature review for version 15.3 is current through August 2007; this topic was last changed on June*27,*2007. The next version of UpToDate (16.1) will be released in March 2008. INTRODUCTION*—*Treatment of iron deficiency anemia must include both attempts to identify and treat the cause of the deficiency (eg, blood loss, poor iron absorption) as well as the administration of iron. The causes and diagnosis of this disorder are discussed separately. (See "Causes and diagnosis of anemia due to iron deficiency"). The diagnosis and treatment of iron deficiency in dialysis patients is a specialized subject, and is presented separately. (See "Iron balance in predialysis, peritoneal dialysis, and home hemodialysis patients" and see "Use of iron preparations in hemodialysis patients" and see "Diagnosis of iron deficiency in chronic kidney disease"). GENERAL PRINCIPLES*—*Oral iron usually provides a safe, cheap and effective means of restoring iron balance. There are a few simple principles governing the use of oral iron [1]: Iron is absorbed best from the duodenum and proximal jejunum. Therefore, the more expensive enteric coated or sustained release capsules, which release iron further down in the GI tract, are counterproductive. Iron salts should not be given with food because the phosphates, phytates, and tannates in food bind the iron and impair its absorption (show table 1). Iron should be given two hours before, or four hours after, ingestion of antacids. Iron is best absorbed as the ferrous (Fe2+) salt in a mildly acidic medium. As a result, we usually add a 250 mg ascorbic acid tablet at the time of iron administration to enhance the degree of iron absorption. The iron preparation used should be based upon cost and effectiveness with minimal side effects. The cheapest preparation is iron sulfate; each tablet contains 325 mg of iron salts, of which 65 mg is elemental iron [2]. Gastrointestinal tract symptoms (eg, abdominal discomfort, nausea/vomiting, diarrhea/constipation) suffered by some patients seem to be directly related to the amount of elemental iron ingested [3]. Thus, the reported low incidence of side effects for some preparations can be explained by their low elemental iron content. For example, a 325 mg tablet of ferrous gluconate contains 36 mg of elemental iron, or 55 percent of the amount of elemental iron in a 325 mg tablet of ferrous sulfate. Patients with persistent gastric intolerance may tolerate ferrous sulfate elixir, which provides 44 mg of elemental iron per 5 mL. Patients can titrate the dose up or down to the level at which the gastrointestinal symptoms become acceptable. ORAL IRON THERAPY*—*A large number of iron-containing preparations are available for the treatment of iron deficiency anemia; some contain other minerals and vitamins, while others may be enteric-coated or have slow-release properties. The latter are especially to be avoided as they may be excreted intact in the stool or release iron too far down the intestinal tract to be maximally effective. A number of factors can inhibit the absorption of iron salts, including the use of antacids, certain antibiotics (eg, quinolones, tetracycline), and the ingestion of iron along with cereals, dietary fiber, tea, coffee, eggs, or milk. Choice of preparation and expected response*—*The most appropriate oral iron therapy is use of a tablet containing ferrous salts, such as: Ferrous fumarate — 106 mg elemental iron/tablet Ferrous sulfate — 65 mg elemental iron/tablet Ferrous gluconate — 28 to 36 mg iron/tablet The recommended daily dose for the treatment of iron deficiency in adults is in the range of 150 to 200 mg/day of elemental iron; there is no evidence that one iron preparation is more effective than another. A single 325 mg ferrous sulfate tablet taken orally three times daily between meals provides 195 mg of elemental iron per day. This regimen should lead to a modest reticulocytosis beginning in approximately seven days and a rise in the hemoglobin concentration of approximately 2 g/dL over the ensuing three weeks. Side effects*—*Approximately 10 to 20 percent of patients may complain of nausea, constipation, epigastric distress and/or vomiting after taking oral iron preparations. There are a number of treatment options for such patients: The patient may take an iron preparation containing a smaller dose of elemental iron (eg, switching from ferrous sulfate to ferrous gluconate), or may switch from a tablet to a liquid preparation, the dose of which (44 mg elemental iron per 5 mL) can be easily titrated by the patient (see "General principles" above). The patient may slowly increase the dose from one tablet per day to the recommended three to four times per day, as tolerated. The iron may be taken with meals, although this will decrease absorption somewhat One or more of these maneuvers should suffice. Parenteral iron therapy should be reserved for the rare patient unable to tolerate even modest doses of oral iron, or in patients whose level of continued bleeding exceeds the ability of the gastrointestinal tract to absorb iron (see "Parenteral iron therapy" below). Duration of treatment*—*There is disagreement as to how long to continue iron therapy: Some physicians stop when the hemoglobin level becomes normal, so that further blood loss will cause anemia and alert the patient and physician to the return of the problem which caused the iron deficiency in the first place Others believe that it is wise to treat for about six months after the hemoglobin normalizes, in order to completely replenish iron stores Our practice is to individualize the duration of iron replacement. As an example, it makes sense to fully replenish iron stores in a patient who became iron deficient as a consequence of multiple pregnancies. On the other hand, we stop therapy once the hemoglobin concentration is normalized in a patient who has occult gastrointestinal bleeding. In this setting, the return of iron deficiency is an important clue that bleeding has recurred. |
|
#6
|
||||
|
||||
|
Failure to respond to oral iron therapy*—*On occasion, a patient may not respond to oral iron therapy. The potential causes for this situation include the following (show table 2): Incorrect diagnosis (eg, thalassemia, myelodysplastic syndrome) Presence of a coexisting disease interfering with response (eg, anemia of chronic inflammation, renal failure). (See "Anemia of chronic disease (anemia of chronic inflammation)", section on Differential diagnosis). Patient is not taking the medication Medication is not being absorbed for physical reasons (eg, enteric coated tablets, concomitant use of antacids). Iron (blood) loss or need is in excess of the amount ingested (eg, severe continuous GI bleeding, dialysis patient, idiopathic pulmonary hemosiderosis). (See "Idiopathic pulmonary hemosiderosis" and see "Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu syndrome)"). The patient has malabsorption for iron. In one study, for example, refractoriness to oral iron treatment was noted in all patients with celiac disease and approximately 70 percent of those with autoimmune atrophic gastritis or Helicobacter pylori infection [4]. (See "Pathogenesis, epidemiology, and clinical manifestations of celiac disease in adults", section on Iron deficiency and see "Metaplastic (chronic) atrophic gastritis", section on Helicobacter pylori).
The appropriate treatment depends upon the cause for failure to respond. Pregnancy*—*Treatment of iron deficiency in pregnancy is the same as that in nonpregnant, postpartum, premenopausal, and postmenopausal women; indications for the use of parenteral iron are also the same [5]. PARENTERAL IRON THERAPY Indications*—*Parenteral iron, which can be given IM but preferably IV, is used in the rare patient who is unable to tolerate even modest doses of oral iron (see "Side effects" above), or in patients whose level of continued gastrointestinal bleeding exceeds the ability of the gastrointestinal tract to absorb iron (eg, hereditary hemorrhagic telangiectasia, iron malabsorption in sprue). Other uses include: Occasional patients with inflammatory bowel disease and iron deficiency may give a history of severe intolerance to oral iron preparations, making therapy with parental iron necessary. (See "Nutritional considerations in inflammatory bowel disease", section on Iron). Parenteral iron is routinely employed in dialysis patients because of ongoing blood loss associated with the procedure, the need for adequate iron to respond to the administration of erythropoietin, and the frequent inability to utilize iron administered orally [6]. (See "Iron balance in predialysis, peritoneal dialysis, and home hemodialysis patients", section on Iron therapy). Parenteral iron has also been employed in anemic cancer patients receiving treatment with erythropoiesis-stimulating agents (eg, erythropoietin, darbepoetin). (See "Role of erythropoiesis-stimulating agents in the treatment of anemia in patients with cancer", section on Supplemental iron). Available preparations*—*There are currently three parenteral iron preparations approved for use in the United States: Iron dextran (INFeD, Dexferrum) preparations contain 50 mg of elemental iron/mL, and can be given either IM or IV. INFeD and Dexferrum differ in that they are "low" and "high" molecular weight dextran preparations, respectively. Both local and systemic side effects can occur following use of iron dextran, with an anticipated frequency of reactions of 4.7 percent [7]. High molecular weight dextran preparations, including Imferon which is no longer manufactured in the United States, are associated with a considerably higher incidence of adverse events than are the low molecular weight preparations [8]. Local reactions include pain, muscle necrosis, and phlebitis in adjacent vessels. Anaphylactic reactions occur in about 1 percent of patients and are thought to be caused by the free iron present in the preparation [7]. Other systemic effects include fever, urticaria, and a flare in arthritis in patients with rheumatoid arthritis. Ferric gluconate complex (Ferrlecit, 12.5 mg iron/mL) Iron sucrose (Venofer, iron saccharate, 20 mg iron/mL) Ferric gluconate complex and iron sucrose are only approved for IV use. (See "Iron balance in predialysis, peritoneal dialysis, and home hemodialysis patients" and see "Use of iron preparations in hemodialysis patients"). Data from Europe and the United States indicate that, in comparison with iron dextran, sodium ferric gluconate complex in sucrose has a reduced incidence of adverse allergic reactions (3.3 versus 8.7 allergic events per one million doses per year), including death (case fatality rate of zero versus 16 percent for allergic/anaphylactic events) [9]. One group has reported on the efficacy and safety of 125 mg of ferric gluconate complex given either undiluted by slow IV push at a rate of 12.5 mg/minute or diluted in 100 mL isotonic saline and infused over 30 to 60 minutes, in 74 patients with iron deficiency anemia and normal levels of serum creatinine [10]. Of 639 infusions, 11 reactions (1.7 percent) were documented; no reaction was considered severe or anaphylactic. Two reactions were moderately severe (eg, dyspnea, severe urticaria, neck and back spasm), causing the infusion to be stopped. All other reactions were minor (eg, pruritus, palpitations, dizziness), causing the infusion to be temporarily stopped, following which all patients were able to continue the infusion. When measured in 56 of the 74 patients, all efficacy end points (eg, increases in hemoglobin, hematocrit, mean corpuscular volume, serum ferritin) showed significant improvement. (See "Iron balance in predialysis, peritoneal dialysis, and home hemodialysis patients" and see "Use of iron preparations in hemodialysis patients"). Iron sucrose also appears to be safe, even among those with a prior history of sensitivity to iron dextran. (See "Iron balance in predialysis, peritoneal dialysis, and home hemodialysis patients" and see "Use of iron preparations in hemodialysis patients"). Intramuscular iron*—*Mobilization of iron from intramuscular sites is slow and occasionally incomplete. As a result, the rise in the hemoglobin concentration is only slightly faster then that which occurs following the use of oral iron preparations. Patients who have very brisk continuing bleeding (as with gastrointestinal angiodysplasia) are difficult to treat with an intramuscular (IM) regimen because repeated courses of IM iron are painful, and the utilization of iron given IM is variable. Patients with severe malabsorption and/or malnutrition may not be candidates for IM treatment if they have a markedly reduced muscle mass into which the iron preparation can be injected. For all of the above reasons, including the fact that IM administration of iron dextran has not been shown to be safer or less toxic than the IV route, the most appropriate parenteral route is the intravenous one [11]. Intravenous iron*—*Intravenous iron is commonly administered to hemodialysis patients because of ongoing blood loss associated with the procedure, the need for adequate iron to respond to the administration of erythropoietin, and the frequent inability to utilize iron administered orally [6]. When ferric gluconate complex is used for this purpose (Ferrlecit®, Schein Pharmaceutical), a test dose of 2 mL of ferrlecit (25 mg elemental iron) is diluted in 50 mL of normal saline and infused IV over 60 minutes. If there is no reaction, up to 125 mg is administered (10 mL) at any one setting, diluted in 100 mL of normal saline, and infused over one hour. The remainder of the calculated dose of iron is given at subsequent dialysis sessions at this rate [12]. (See "Iron balance in predialysis, peritoneal dialysis, and home hemodialysis patients", section on Iron therapy). Calculation of the dose*—*In selecting the dose of parenteral iron required, one calculates the iron deficit based upon the fact that 1 gm of hemoglobin contains 3.3 mg of elemental iron (show table 3). Assume, for example, that a 60 kg woman with a hemoglobin concentration of 8 g/dL due to iron deficiency needs parenteral iron replacement, which will be given intravenously in the form of iron sucrose (20 mg iron/mL). The normal blood volume is approximately 65 mL/kg. Thus, her total blood volume is approximately 3900 mL or 39 deciliters (65 mL/kg x 60 kg). A normal hemoglobin concentration would be 14 g/dL. Thus, her hemoglobin deficit is 6 g/dL with a total deficit of 234 g (6 g/dL x 39 dL). Each gram of hemoglobin contains 3.3 mg of iron. Thus, her total red cell iron deficit is 772 mg (234 g of hemoglobin x 3.3 mg Fe per gram of hemoglobin). At an iron concentration of 20 mg/mL, this would require a total of 38.6 mL of iron sucrose. The product information sheet, as well as institutional guidelines, should be consulted for the need for a test dose, proper dilution, rate of infusion, and maximal allowable dose per infusion |
|
#7
|
||||
|
||||
|
Diagnosis and treatment of iron deficiency without anaemia
Тезисы немецкоязычной статьи из клиники, где ранее работал (выделил мне ранее неизвестные моменты):
Praxis (Bern 1994). 2009 Dec 2;98(24):1445-51. Diagnosis and treatment of iron deficiency without anaemia. Fehr J, Krayenbühl PA, Favrat B, Schleiffenbaum B, Kapanci C, Orelli F. vormals Klinik für Hämatologie. Iron deficiency (ID) without anaemia frequently remains undiagnosed when symptoms are attributed to ID with anaemia. Serum ferritin is the primary diagnostic parameter, whereas <10 mug/l represent depleted iron stores, 10-30 mug/l can confirm ID without anaemia and 30-50 mug/l might indicate functional ID. In case of increased CRP or ALT, normal/elevated ferritin should be interpreted with caution. Intravenous iron is indicated if oral iron is not effective or tolerated. At ferritin <10 mug/l, a cumulative dose of 1000 mg iron and at ferritin 10-30 mug/l, a cumulative dose of 500 mg is advised. At ferritin 30-50 mug/l a first dose of 200 mg might be considered. Ferritin shall be reassessed not sooner than 2 weeks after the last oral or 8-12 weeks after the last iv iron administration. |






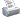

 Линейный вид
Линейный вид

